Background
In November 2022 Epilepsy Action, Young Epilepsy and Epilepsy Society carried out a survey of women and girls with epilepsy, and their parents and carers, asking them about the information they have received around the risks of taking some epilepsy medicines during pregnancy.
Previously, in 2017 and 2019, these charities conducted a survey of women and girls with epilepsy who take valproate as a medicine, and their parents and carers.
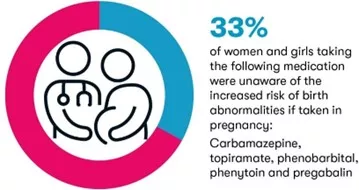
While the risks around valproate taken in pregnancy have been known for some years, some other epilepsy medicines also pose a risk if taken during pregnancy. A 2021 safety review of epilepsy medicines used during pregnancy carried out by the Medicines and Healthcare products Regulatory Agency (MHRA) showed that a number of epilepsy medicines were linked with an increased risk of birth abnormalities if taken during pregnancy, including carbamazepine, phenobarbital, phenytoin and topiramate.
While none of the medicines posed as much of a risk as valproate, using carbamazepine (brand names Curatil, Tegretol), phenobarbital (brand names Phenobarbital Accord, Phenobarbital Elixir) or topiramate (brand name Topamax) during pregnancy increases the risk of physical birth abnormalities compared with the general population.
This survey looked to measure current levels of awareness around the risks of valproate, as well as the levels of awareness around the other epilepsy medicines.
This report explains the findings of the survey, based on the 1269 responses we received from women and girls with epilepsy and their parents and carers.
Key Findings
Awareness of risks
“We were not made aware of the dangers of some of the medications talked about in this survey, we were told about epillim [valproate]. The information was scant”.
The survey found that while there has been an increase in the proportion of people who are aware of the risks of valproate compared to 2017 & 2019, a significant number of women and girls were unaware of the risks of other epilepsy medicines.
I know I’m only 13 and do not plan on children for many years but I don’t know if any of these medications may mess things up for me in the future. No one tells you anything really.
Table 1*
| Medication | Percentage of people unaware of the risks relevant to their medication |
| Valproate | 9% |
| Carbamazepine | 35% |
| Phenobarbital | 75% |
| Phenytoin | 50% |
| Pregabalin | 35% |
| Topiramate | 28% |
- 9% of women taking valproate didn’t know that taking it during pregnancy can increase the risks of serious birth defects or learning and development problems in children.
- 33% of women taking carbamazepine, topiramate, pregabalin, phenytoin, or phenobarbital didn’t know they increase the risk of physical birth abnormalities (if taken during pregnancy)
- 58% of women taking clobazam, gabapentin, oxcarbazepine and zonisamide didn’t know there is currently not enough research to make any firm conclusions about their safe use during pregnancy
- Overall, 68% of women were aware of the risk information related to their medication
When I was looking into getting pregnant I was on Epilim [ valproate]. I was then switched to Carbamazepine - but I had no idea that this also caused birth defects.
*It should be noted that for phenobarbital, phenytoin and pregabalin the sample sizes were quite small (4, 20 and 34 respectively)
Discussions with healthcare professionals
The majority of women and girls had discussed the risks of taking epilepsy medicines during pregnancy, with most of these discussions taking place with either a neurologist, paediatrician or epilepsy specialist nurse. However, there are several aspects that influence whether the individual would expect to have had a discussion, including pressures and limitations on healthcare professionals that mean these conversations are not happening.
- 31% of women have not had a discussion with a healthcare professional about the risks associated with taking epilepsy medicines during pregnancy
- 25% of women have discussed the risks associated with taking epilepsy medicines during pregnancy with a GP in the last 12 months
- 49% of women have discussed the risks associated with taking epilepsy medicines during pregnancy with a neurologist/pediatrician in the last 12 months
- 9% of women have discussed the risks associated with taking epilepsy medicines during pregnancy with a pharmacist in the last 12 months
- 42% of women have discussed the risks associated with taking epilepsy medicines during pregnancy with an Epilepsy Specialist Nurse in the past 12 months
- Overall, 31% of women are not satisfied with discussions with their healthcare professionals about the risks associated with taking epilepsy medicines during pregnancy
The way the information is presented to us can sometimes make us feel like our needs are not as important as the health of the pregnancy, this happens a lot during GP visits with young adults.
Satisfaction with the information received
I was satisfied until I did this survey and it seems now I missed out on information for example I was not aware about the effects with taking carbamezapine.
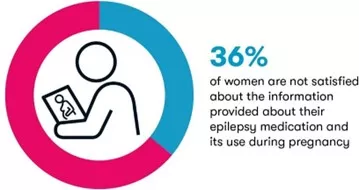
- Overall, 36% of women are not satisfied about the information provided about their epilepsy medication and its use during pregnancy (17% are not at all satisfied)
- However, 84% of respondents were satisfied with the level of information about valproate
- Women and girls who have spoken with a healthcare professional were satisfied with the discussion about the risks associated with taking epilepsy medicines during pregnancy (81% satisfied)
Regional results
Comparing the results across different regions shows that awareness levels in England are higher than the other nations, though satisfaction levels with the information provided are similar, with the exception of Northern Ireland. However, there are also areas of England that are performing worse than others in certain aspects.
Table 2
| Region | Awareness of risks related to valproate | Awareness of risks related to other ASMs | Overall satisfaction with information received |
| UK | 91% | 67% | 64% |
| England | 93% | 70% | 65% |
| Northern Ireland | 80% | 50% | 54% |
| Scotland | 86% | 50% | 65% |
| Wales | 81% | 69% | 65% |
- 50% of women in Northern Ireland and Scotland didn’t know that using carbamazepine, topiramate, pregabalin, phenytoin, or phenobarbital during pregnancy increases the risk of physical birth abnormalities
- 45% of women in the North West of England didn’t know that using carbamazepine, topiramate, pregabalin, phenytoin, or phenobarbital during pregnancy increases the risk of physical birth abnormalities
- Women in Northern Ireland and the North West and South East of England have the lowest rates for discussions with a professional healthcare about the risks of epilepsy medications and pregnancy (around 37% have not had a discussion)
- In Northern Ireland and the South West of England, 28% women are satisfied with the discussions with their healthcare professional about the risks associated with taking epilepsy medicines during pregnancy, compared to the 39% in Scotland and 38% in the South East of England
- 13% of women in London are not very satisfied with their discussion with their healthcare professionals, likewise with 14% of women in Northern Ireland and the South East of England and 15% in the South West of England. In comparison, 6% of women in the North East of England and Yorkshire answered the same
Results by age groups
I am 23 and have not had anybody speak to me about epilepsy and pregnancy in the 16 years of me having epilepsy.
The results of the survey showed a huge gap in the levels of awareness of the risks of using carbamazepine, phenobarbital, phenytoin, pregabalin or topiramate during pregnancy among those under 24 years of age, with more than half of under 24s being unaware of the risks compared to around a quarter of those over 24.
Table 3
| Age | Awareness of risks related to valproate | Awareness of risks related to other ASMs | Overall satisfaction with information received |
| Under 24 | 95% | 47% | 61% |
| 25-44 | 98% | 71% | 66% |
| Over 45 | 80% | 74% | 65% |
- 20% of women over 45 didn’t know valproate during pregnancy can cause serious birth defects or learning and development problems in children
- 2% of women between 25-44 didn’t know valproate during pregnancy can cause serious birth defects or learning and development problems in children
- 5% of women under 24 didn’t know valproate during pregnancy can cause serious birth defects or learning and development problems in children
- 53% of women under 24 didn’t know that using carbamazepine, phenobarbital, phenytoin, pregabalin or topiramate, during pregnancy increases the risk of physical birth abnormalities, compared to 29% of women between 25-44 and 26% of women over 45
Conclusions
While it is very encouraging to see the increased awareness of the risks of taking valproate during pregnancy (with only 9% of women unaware of the risks, compared to 18% in the 2019 survey), it is clear that more needs to be done to communicate the risks that other epilepsy medicines pose if taken during pregnancy.
A review of the use of epilepsy medicines in pregnancy carried out by the Medicines and Healthcare products Regulatory Agency (MHRA) and Commission on Human Medicines (CHM) identified that medicines other than valproate can be harmful to the unborn child if taken during pregnancy.
Despite this review being published two years ago, not enough has been done to communicate these findings to women and girls with epilepsy, and healthcare professionals. The fact that a third of women taking such medicines were unaware of the risks is of huge concern.
The CHM review also identified that for a significant number of epilepsy medicines, there is not enough information about whether or not they are safe to use during pregnancy. Again, many people responding to the survey were unaware of this, with over half (58%) of respondents taking these medicines saying they did not know. It is very concerning that so many women and girls with epilepsy are taking medications when the risks of their use during pregnancy is unknown. It cannot be acceptable that people with epilepsy do not have the information about whether or not their medication is safe to use during pregnancy. We urgently need research into these medications so that women and girls can be made aware of their potential risks and make an informed choice about their own treatment.
There is also a clear disparity between the levels of satisfaction with the information people are given about the risks of valproate, and that provided about other epilepsy medications.
Too many women also reported not having a discussion with a healthcare professional about the risks associated with taking epilepsy medicines during pregnancy. While thankfully these discussions are happening more frequently around valproate, more still needs to be done to ensure that healthcare professionals are having these discussions with women and girls on all epilepsy medicines. Women and girls and their parents and carers need to be given the information to make an informed choice about their treatment.
In the responses to the survey, we also heard many stories from families of children that had been harmed by valproate:
“I received no information and no follow up support or ongoing support despite my daughter having issues associated with valproate and despite me raising the issues with doctors and consultants”.
“Was told to continue taking my epilim through both my pregnancies which have had consequences on both my children.”
“The information came a little too late for me as I was one of the ladies whose son suffered 18 yrs ago…He is now 18 but has suffered terribly in life due to a diagnosis of ASD, his emotions can be very hard to handle…I can never prove whether it was or wasn’t the medication, deep down I always blamed valproate. I’m extremely upset it took this long to identify the drug to be harmful to our unborn children as many children like my son will have to sadly struggle in life with a disability that could have been prevented.”
We also heard from women who have had to make difficult decisions about starting a family due to valproate:
“I was told I had to stop taking valproate and onto Keppra [Levetiracetam]. So I did, over a 12 month period I had several seizures and was hospitalised. This resulted in me going back on valproate. I have tried other medications and they do not work apart from valproate. I have always been given enough info about the risks of having children on valproate which I understand. However, there is no information out there about what to do next. I.e, surrogacy or other routes.”
“I wish I was informed earlier about the risks. My plans for pregnancy have been put on hold for a long time and if I’d known sooner at a younger age I could have changed medication sooner and possibly have a family of my own by now.”
“Its possible side effects have been explained numerous times by GP & several pharmacists but as this is the only medication that works & I am not planning a pregnancy I chose to remain on it.”
“This was not explained when I was first put on valproate. As I am now in the position where no other medicines will work, I have decided to not have a baby. This has been extremely upsetting and has greatly affected my life and relationship. I would not have tried valproate if I had been provided this info”
However, there also remains an issue around the use of valproate by women and girls with learning disabilities for whom pregnancy is highly unlikely. We have heard from many parents that repeated conversations around this topic are extremely distressing:
“I am sick to death of hearing about this. My daughter does not have the capacity to consent to sex. It could only be rape…I find this constant talk about valproate, to my daughter at the level of a two year old, to be rude and intrusive. She would like a baby, but she has no comprehension of how to look after one. She can’t look after herself. All it does, is raise questions that are painful for her and us.”
“Young person has learning disability and extremely unlikely to be in a situation where pregnancy would be a risk…The whole discussion around pregnancy and valproate was inappropriate almost created a barrier to accessing a beneficial drug and caused more stress and paperwork for our family. I fully support education around potential damage to the unborn child but there needs to be some common sense too and not make life more difficult for families.”
Recommendations
From the results, it is clear that there is still an ongoing requirement to communicate the risks associated with not only valproate, but all anti-seizure medications (ASMs) during pregnancy.
The availability of accessible, resourced and structured pre-conception counselling has the ability to support patient safety and make significant decisions relating to pregnancy.
“I had pre - pregnancy counselling with my neurologist years ago when I was planning to get pregnant. He had always told me to come and discuss it with him first, when I was much younger (in my twenties). We always said it took 3 of us to make our baby due to the medication planning that went on beforehand!”
We also urgently need more research into epilepsy medicines so that women and girls can make an informed decision about their treatment.
“Whilst my Professor, GP and Epilepsy Nurse have been incredibly helpful I still feel like there is some information that they are not able to provide due to not knowing themselves. More research needs to be completed so we can give people with epilepsy definite answers and information.”
Giving consideration to the findings, and evaluating the current strategies which are not working, we are making the following recommendations for action to address sustained quality and safety for those people of child baring age and to support them in making informed decisions supported by healthcare professionals:
To make any significant changes requires the healthcare system leaders and key stakeholders as identified against each recommendation to commit to:
- A national review of pre-conception counselling services, this supports areas demonstrating health inequalities for people of child-bearing potential to make significant and appropriate life choices. That they are provided with the right information at the right time by the right healthcare professional. (ACTION - NHS - Patient Safety, & MHRA).
- Develop a repository of information and resources readily available with appropriate sign posting, has national backing and is available to both healthcare professionals and service users. That this is used as a learning and sharing platform e.g. Neurological Alliance Tool Kit safety, how to use the Annual Risk Acknowledgement Form (ARAF), EpSMon tool, SUDEP, drug risks and benefits. (ACTION - NHS – Patient Safety & MHRA).
- Commissioners fully understanding what services they have and are responsible for in relation to their capacity and allocation of resources e.g. Neurology/ Epilepsy services and appointment capacity, Epilepsy Specialist Nurse recruitment and service delivery, GP/ family planning services, and include these in service specifications. To monitor and discuss user experience and share the learning back into service evaluations. (ACTION - Integrated Care Systems / Integrated Care Boards / Health Care Partnerships / NHS Services).
- National commitment to fund research, there is not nearly enough available research and available information on the effects of taking ASMs on pregnancy. This should include supporting work being carried out by epilepsy charities, such as Epilepsy Research UK’s research priorities, Safe Mum Safe Baby (Epilepsy Society), and Priority 8 of the UK Epilepsy Priority Setting Partnership (PSP)2 (Epilepsy Action). (ACTION - NHS, MHRA)

